English Page
Welcome to Agata Lab Homepage in Department of Biochemistry and Molecular Biology, Shiga University of Medical Science (SUMS)!

Prof. Yasutoshi Agata
Our research focuses on how cancer cells are developed and how our immune cells fight against cancers. We are currently conducting four projects described below. We are seeking for students who are willing to perform these research projects with us as a graduate student. Please feel free to contact me by email (yagata*belle.shiga-med.ac.jp Please change * to @.) if you are interested.
1) Regeneration of T cells that fight against cancers from iPS cells
In cancer immunotherapies, it is difficult to propagate tumor-specific T cells obtained from cancer patients. To overcome this problem, we have tried to regenerate T cells that can fight against cancers from iPS cells (iPSCs) in collaboration with Dr. Hiroshi Kawamoto in Kyoto Univ, who made iPSCs from tumor antigen-specific T cells (T-iPSCs) and then regenerate T cells from the T-iPSCs(Cell Stem Cell 12: 31, 2013). We have developed methods to regenerate T cells efficiently and rapidly by introducing tumor antigen-specific T-cell receptor (TCR) genes into iPSCs using lentiviruses and succeed to regenerate T cells that can kill tumor cells.
Furthermore, we have developed a novel method by which TCR genes can be efficiently knocked-in into the endogenous TCRβ locus using genome editing and cassette exchange, to elevate the TCR expression level and avoid random integration of the vector, which might cause tumorigenesis.
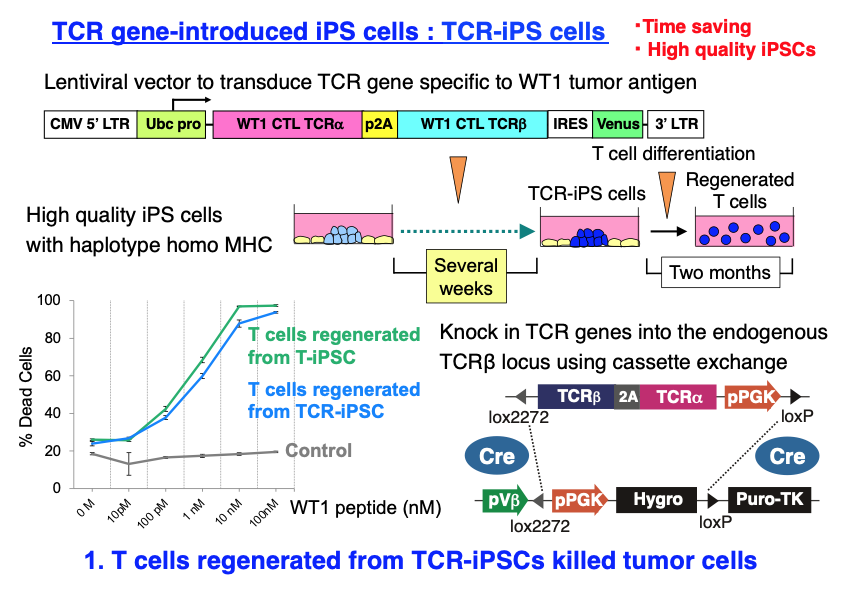
Maeda T, Nagano S, Kashima S, Terada K, Agata Y, Ichise H, Ohtaka M, Nakanishi M, Fujiki F, Sugiyama H, Kitawaki T, Kadowaki N, Takaori-Kondo A, Masuda K, Kawamoto H.* Regeneration of tumor antigen-specific cytotoxic T lymphocytes from iPSCs transduced with exogenous TCR genes. Molecular Therapy - Methods & Clinical Development, 19:250-260, 2020.
Kashima S, Maeda T, Masuda K, Nagano S, Inoue T, Takeda M, Kono Y, Kobayashi T, Saito S, Higuchi T, Ichise H, Kobayashi Y, Iwaisako K, Terada K, Agata Y, Nakamura K, Saito M, Narita S, Ogawa O, Habuchi T, Kawamoto H. Cytotoxic T lymphocytes regenerated from iPS cells have therapeutic efficacy in a patient-derived xenograft solid tumor model. iScience 23(4): 100998, 2020
2) Isolation of TCR genes with tumor-killing activity from tumor-infiltrating T lymphocytes (TIL) in cynomolgus monkeys
To evaluate the efficacy of cancer immunotherapies, we need primate animal models because substantial differences between the mouse and human immune systems hamper the clinical translation of the results obtained in mouse tumor models. We have thus used a macaque model in which tumor cells are transplanted into MHC-matched monkeys. We cloned TCR genes from single tumor-infiltrating lymphocytes (TIL) and successfully identified TCR genes which can kill tumor cells upon introduction in T cells.
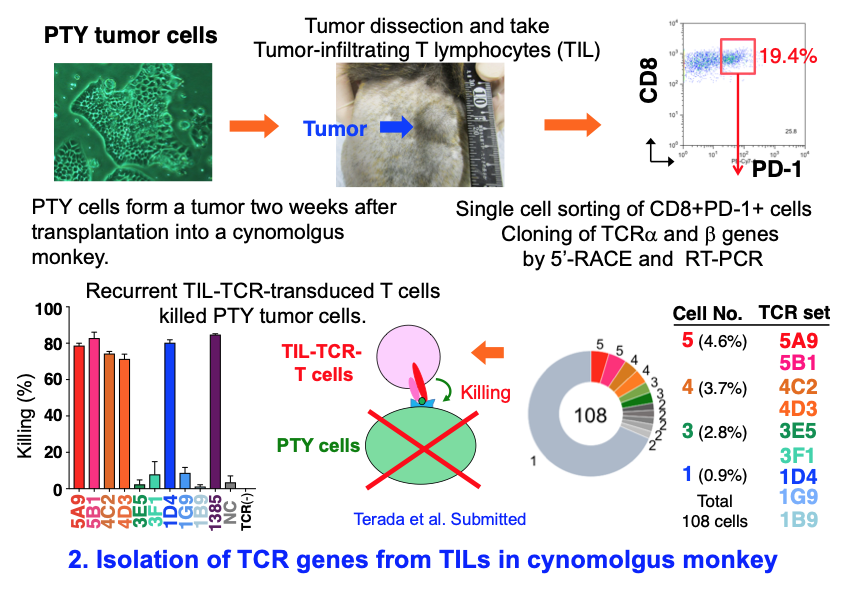
Terada K, Kondo K, Agata Y, et al. Submitted
Satooka H, Ishigaki H, Todo K, Terada K, Agata Y, Itoh Y, Ogasawara K, Hirata T. Characterization of tumour-infiltrating lymphocytes in a tumour rejection cynomolgus macaque model. Sci Rep. 10(1):8414, 2020
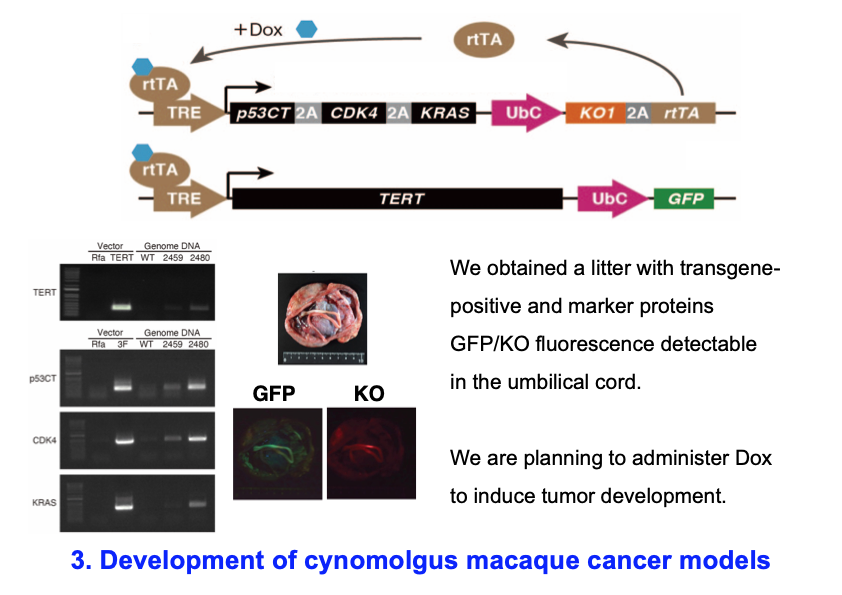
3) Development of cynomolgus macaque cancer models
The mechanisms underlying cellular transformation are different between rodents and primates. For example, in mice, inactivation of one tumor suppressor gene such as p53 and activation of one oncogene such as Kras are sufficient to develop tumors in many cancer models. In contrast, forced expression of at least four cancer-inducing genes is required to convert normal human cells into tumorigenic ones. We have thus tried to develop macaque models in which four cancer-promoting genes are inducibly expressed by Doxycycline (Dox). We succeeded to obtain a litter with transgene-positive and marker proteins GFP/KO fluorescence detectable in the umbilical cord. We are planning to administer Dox to induce tumor development. If cancer develops in this monkey, it will be the first cancer model monkey in the world.
4) Investigation of super-enhancer functions in cancer development
In cancer cells, cancer-promoting genes are highly expressed by the formation of super-enhancers (SEs), large clusters of enhancers. Bromodomain and extra terminal domain (BET) protein BRD4 binds to SEs that drive high expression of oncogenes in many cancers. A BET inhibitor JQ1 has been found to suppress malignant phenotypes of cancer cells, however, the target genes of JQ1 remain largely unknown. We identified the long non-coding RNA MANCR, which is markedly down-regulated by JQ1, and found that BRD4 binds to the MANCR locus and MANCR plays a critical role in migration and invasion of cancer cells.
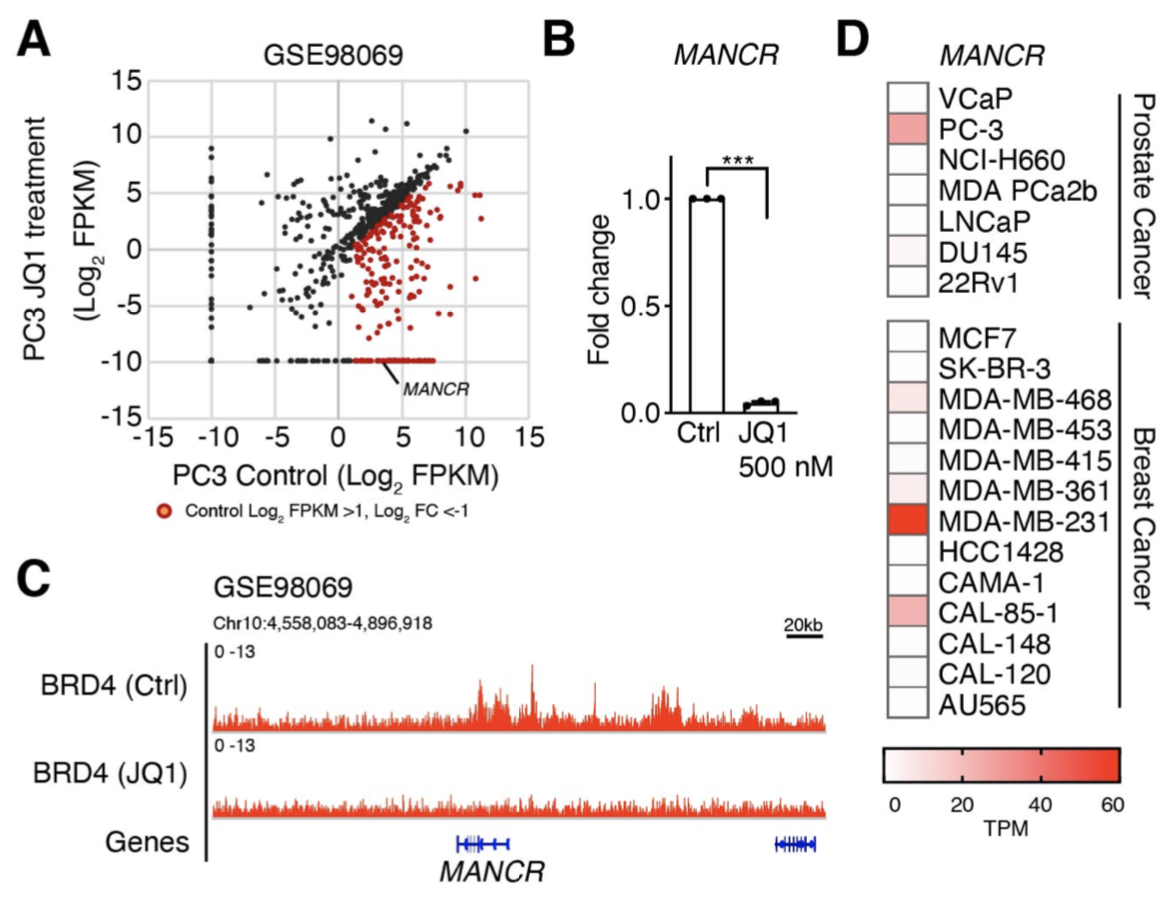
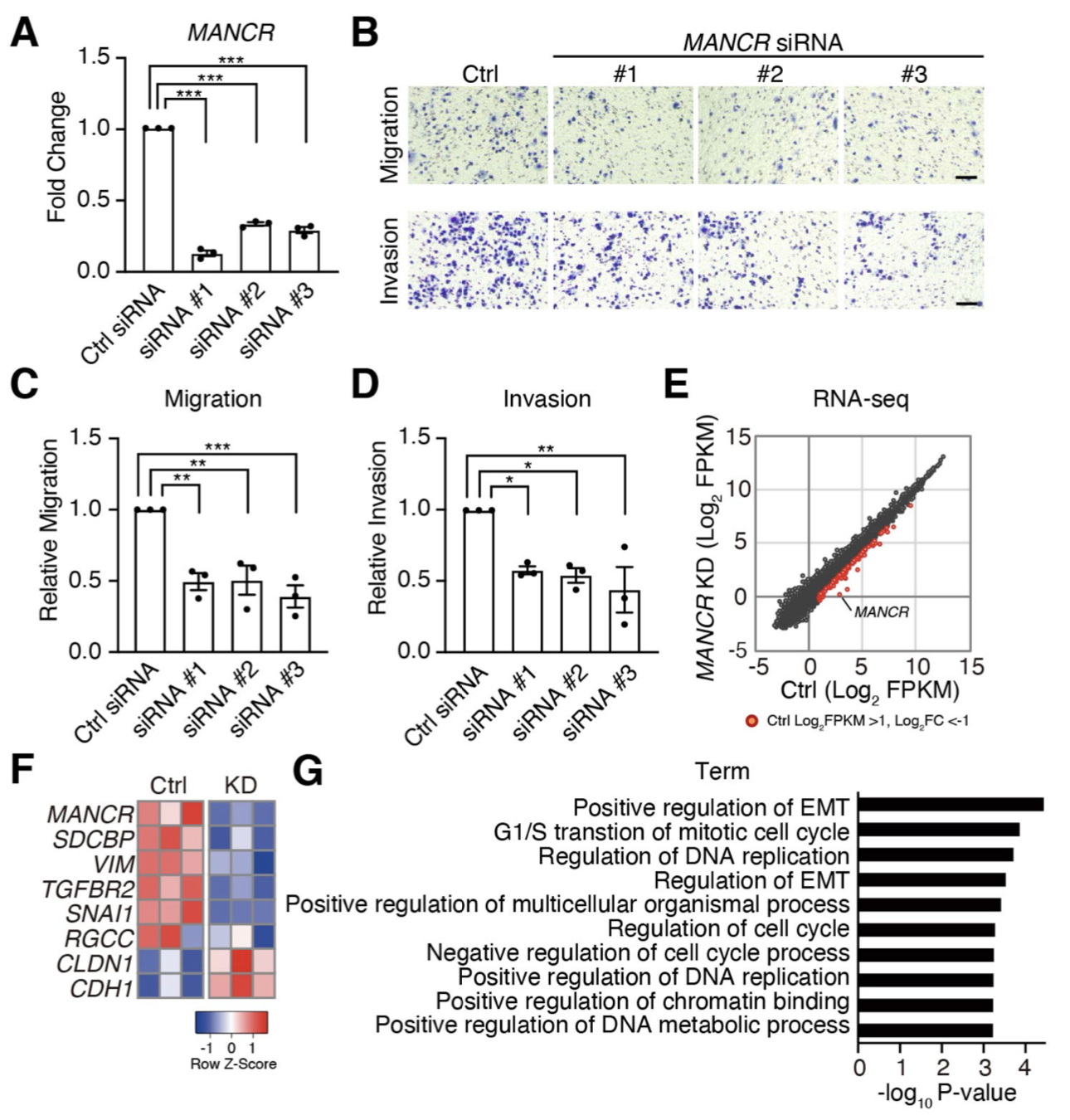
Nagasawa M, Tomimatsu K, Terada K, Kondo K, Miyazaki K, Miyazaki M, Motooka D, Okuzaki D, Yoshida T, Kageyama S, Kawamoto H, Kawauchi A, Agata Y. Long non-coding RNA MANCR is a target of BET bromodomain protein BRD4 and plays a critical role in cellular migration and invasion abilities of prostate cancer. Biochem Biophys Res Commun. 526, 128-134, 2020

with Drs. Tasuku Honjo and Yasumasa Ishida