Research Map
Current Research Projects
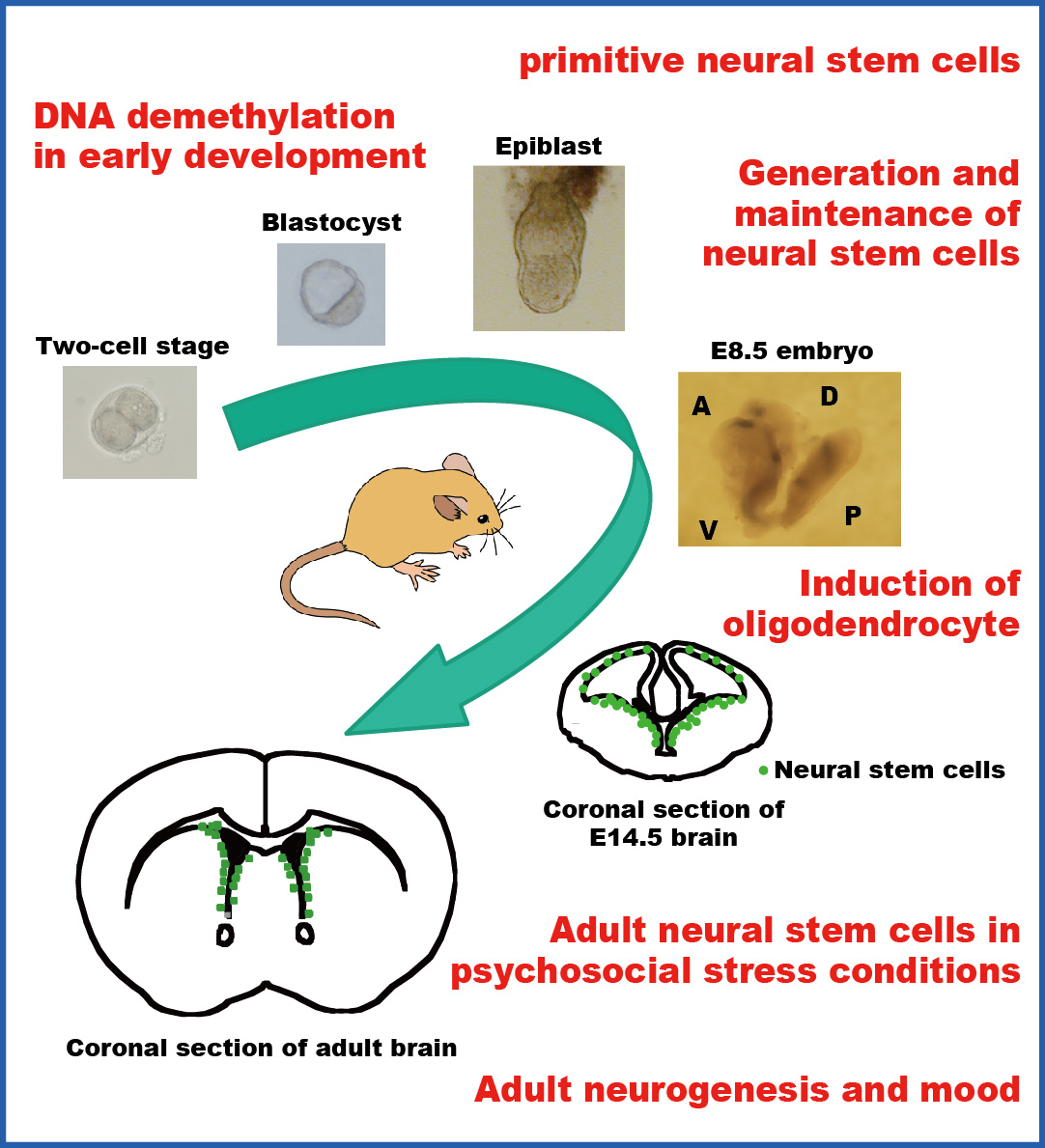
We previouly reported that Notch receptor-mediated signaling plays critical roles in the induction and maintenance of neural stem cells and that activation of Notch signaling is first detectable by the expression of an effector gene, Hes5, in the neuroepithelium of E8.0–8.5 mouse embryos (Genes & Dev 2004). Recently, we show that mammalian Glial cells missing (Gcm) 1 and 2 are involved in the epigenetic regulation of Hes5 gene transcription by DNA demethylation in a DNA replication-independent manner. Loss of both Gcm genes and subsequent lack of Hes5 upregulation in the neuroepithelium of E7.5–8.5 Gcm1,Gcm2 double null mutants results in the impaired induction of neural stem cells. We suggest that Hes5 expression is serially activated first by Gcms and later by the canonical Notch pathway. These results were published in Nature Neuroscience 2011.
Recently, we found that Gcm1 is involved in gliosis after brain injury through its demethylation activity (iScience 2025). Gcm1 is transiently upregulated as early as 8 hours after the injury and induces Lif and Vegf genes, leading to gliosis and angiogenesis in the penumbra of the lesion.
We have also explored the histone modification that is involved in the activation of Notch signaling. We recently found and reported that Bre1a, histone H2B-specific E3 ubiquitin ligase, plays a significant role in the maintenance of neural stem cells and determination of differentiation timing by regulating the expression of Hes5 and p57Kip2 genes (J Neurosci 2014).
When GFP-expressing lentivirus infects neural stem cells (NSCs), its genome is randomly integrated into the host genome. By identifying the integration sites in the GFP+ neurosphere-forming NSCs, we traced their progeny in the olfactory bulb (OB) and the cortex of the adult brain by integration site-specific PCR. We revealed unique properties of NSCs (Cerebral Cortex 2020, J Physiol Sci 2023), which challenges a traditional view of NSCs in the adult brain. We propose that life-long quiescent NSCs segregate into several types in the early embryogenesis: Cortex-NSCs, OB-NSCs, and double negative (DN)-NSCs. Importantly, we found that most of NSCs in the subependymal zone are consumed after providing new neurons into the OB.
The Lewis X carbohydrate antigen is abundantly expressed in several stem cell populations and is considered to play a critical role in the maintenance of self-renewal of stem cells. Attachment of fucose to a non-reducing terminus of sugar chains of glycoproteins and glycolipids could alter the cell-cell recognition, cell migration, and intercellular signal transduction. We have identified a novel α1,3-fucosyltransferase, Fut10, and showed that Fut10 is responsible for the synthesis of unique types of Lewis X on N-glycans and alters stem cell functions (J Biol Chem 2013). It would be intriguing to further study the function of Fut10 and its specific substrate glycoproteins.
We are interested in how pluripotent stem cells, whic are found in blastocysts or epiblasts in the early mammalian development and are equivalent to ES cells, are induced to generate neural stem cells. We have developed a serum-free culture system, in which ES cells differentiate into primitive neural stem cells in the presence of LIF (Neuron 2001). Primitive neural stem cells express neural precursor markers, generate neurons and glia in vitro, and have neural and non-neural lineage potential in vivo. Primitive neural stem cells are not only induced in vitro from ES cells but also exist in the epiblast (Genes & Dev 2004).
In rodents, neural stem cells are stably maintained in the subependyma and provide new neurons in the olfactory bulb and the dentate gyrus of the hippocampus. We studied neural stem cells in mice that were exposed to chronic stress by forced swimming and found that chronic stress decreased the number of neural stem cells in the subependyma. Interestingly, the reduction of neural stem cell number persisted for weeks after the cessation of stress, but was reversed by treatment with antidepressant drugs. We demonstrated that serotonin increased the survival of neural stem cells in in vitro colony-forming neurosphere assay and that serotonin expanded the size of the neural stem cell pool in the subependyma when it was infused into the lateral ventricle in vivo (J Neurosci Res 2007).
We have screened drugs that are used to treat neurological and psychiatric diseases and found that mood stabilizing drugs, which are used to treat patients with bipolar disorder, enhance the self-renewal capability of mouse neural stem cells in vitro. Importantly, this enhancement is achieved at therapeutically relevant concentrations in the cerebrospinal fluid. The pharmacological effects are mediated by the activation of Notch signaling in the neural stem cell. We demonstrated that chronic administration of mood stabilizers expanded the neural stem cell pool in the adult brain, which subsequently increased the cell supply to the olfactory bulb (Stem Cells 2008).