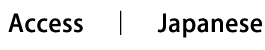The immune response is a critical component of host defense against infection and is essential for normal health. Unfortunately, immune responses are also sometimes directed to our own cells and tissues, leading to autoimmune diseases, and to inherently harmless environmental antigens, causing allergy. The rates of these diseases are increasing worldwide, demanding a major effort toward prevention and control in the 21st century. However, clear therapeutic strategies for these diseases have not been established.
The immune system is different from other organ systems in that most cells in the system are migratory; the cells do not stay in one particular tissue but move from one tissue to another. The migratory behavior of immune cells has a crucial role in immune responses. Our laboratory’s goal is to understand how immune cells migrate in the body during health and disease. The ultimate goal is to utilize the knowledge we gain through our research in the development of effective therapies for allergic and autoimmune diseases.
1. Mechanisms of immune cell trafficking
As mentioned above, immune cells traffic in the body, and their migratory patterns are different depending on the cell types. For example, naive lymphocytes recirculate from the blood, through lymphoid organs such as lymph nodes and Peyer’s patches, into lymph, and back to blood. In contrast, antigen-stimulated lymphocytes migrate to peripheral tissues such as skin and mucosa, where they act. We study the mechanisms by which immune cells recirculate and migrate to specific sites. In particular, we are investigating how lymphocytes migrate to the skin and mucosa, focusing on the roles of adhesion molecules and chemokines. The skin and mucosa are the principal physical barriers from pathogens, and it is crucial for host defense that pathogen-specific lymphocytes are efficiently recruited to these sites. On the other hand, lymphocyte localization to skin and mucosa plays a key role in the pathogenesis of many inflammatory skin diseases such as atopic dermatitis, nasal allergies, as well as intestinal inflammatory diseases such as Crohn’s disease and ulcerative colitis. Based on the understanding of molecular mechanisms of lymphocyte migration to the skin and mucosa, we aim to develop strategies for controlling immune cell trafficking for the treatment of cutaneous, nasal, and intestinal inflammatory diseases.
Reference : J Immunol 178, 2499-2506, 2007
2. Immune regulation by ERM proteins
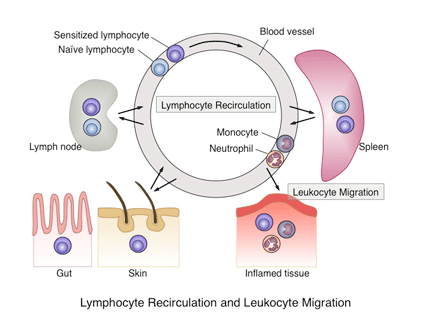
It is well established that cytoskeletal proteins are essential for determining cell shape and regulating cell adhesion, motility, and movement. The ezrin–radixin–moesin (ERM) proteins are a family of membrane-associated proteins that link membrane proteins to the cortical cytoskeleton, thereby regulating the structure and function of specific domains of the cell cortex. Our laboratory has shown that mice deficient in moesin exhibit lymphopenia in the peripheral blood and that this is at least partly caused by impaired lymphocyte egress from the thymus, bone marrow, and peripheral lymphoid organs. Recent work in our laboratory has also shown that these mice exhibit various defects in immune responses. We are currently exploring the roles of immune cell dynamics regulated by the ERM protein moesin in the control of immune responses. Our goal is to exploit the knowledge on ERM regulation of cell dynamics for controlling immune responses in various immune-related diseases.
Reference : Int Immunol 24, 705-717, 2012
3. GPCR signaling in the immune system
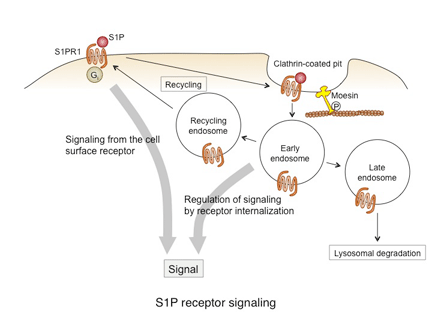
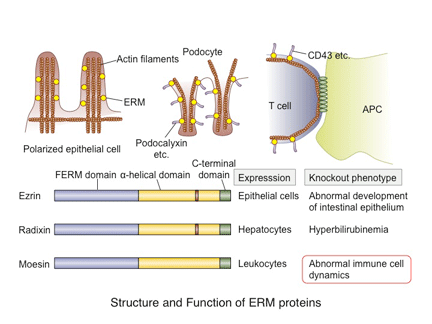
Multiple families, each with a distinct function, provide the traffic signal for immune cells. Adhesion molecules, such as selectins and integrins, are key determinants for the destination of cell trafficking. The expression of specific adhesion molecules and the regulation of their activity in immune cells as well as in target tissues regulate immune cell migration and localization. Other important factors determining the cell trafficking include chemokines and lipid mediators. These factors bind to specific G protein-coupled receptors (GPCRs) and activate signaling cascades mostly through heterotrimeric G proteins. It has become clear in recent years that multiple signal transduction pathways are employed upon GPCR activation and that these pathways are regulated by various events after ligand binding. Many GPCRs are internalized from the cell surface upon ligand binding, which regulates signaling through the receptor. In the immune system, such receptor dynamics are closely related to immune cell function and trafficking. In particular, signaling through the receptor for sphingosine 1-phosphate (S1P), S1PR1, is tightly controlled by receptor internalization. Recent studies from our group indicate that the ERM protein moesin regulates S1PR1 internalization. We are currently exploring the mechanisms underlying the internalization of GPCRs including S1PR1 and the regulation of signaling by receptor internalization.
Reference : PLoS One 16, e82590, 2013