Development of vaccines and therapeutic agents against influenza virus infection
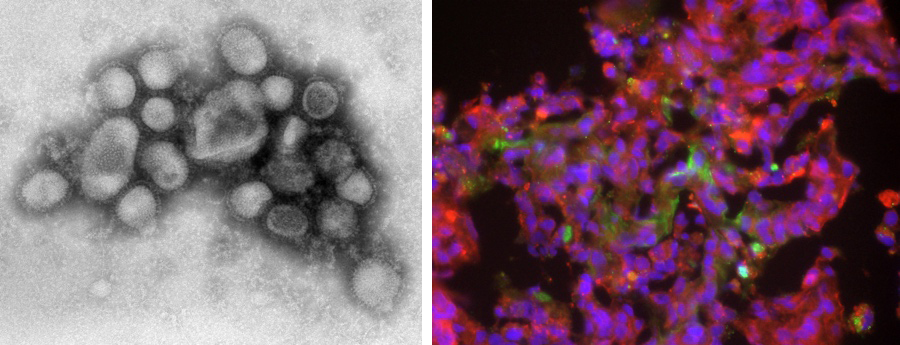
Using cynomolgus monkeys, we clarified the pathogenicity of the pandemic H1N1 influenza virus that was prevalent worldwide in 2009. We have also clarified the pathogenicity of highly pathogenic avian influenza virus (HPAIV) since HPAIV infection in birds and humans is sporadically reported around the world. Therefore, we have established an animal experimental model in which cynomolgus macaques show a severe disease after HPAIV infection. Using this model, the cause of the aggravation in cynomolgus monkeys after HPAIV infection has been revealed.
We verify the efficacy and safety of the whole virus particle vaccine against influenza virus with an improved inactivation method, which were developed in collaboration with Professor Hiroshi Kida in Hokkaido University using not only mice but also cynomolgus monkeys. In addition, we are analyzing the mechanism of protective immunity that the whole virus particle vaccine induces.
In the research on treatments for influenza virus infection, we use the monkey model to verify the efficacy of existing and newly developed drugs. Furthermore, using animal models, we predict the frequency of drug-resistant viruses and analyze the properties of resistant viruses.
Articles
Pathogenicity
- Antiviral Research 178: 104790, 2020. doi.org/10.1016/j.antiviral.2020.104790
- Virology 493: 31-38, 2016. doi: 10.1016/j.virol.2016.03.007.
- PLOS One 8: e75910, 2013. doi.org/10.1371/journal.pone.0075910
- Virology 407: 178-184, 2010. doi: 10.1016/j.virol.2010.08.006.
- Nature 460: 1021-1025, 2009. doi: 10.1038/nature08260.
Vaccines
- npj Vaccines 10:164, 2025. doi.org/10.1038/s41541-025-01221-x
- Vaccine 40: 4026-4037, 2022. doi.org/10.1016/j.vaccine.2022.05.045
- Vaccine 35: 1008-1017, 2017. doi: 10.1016/j.vaccine.2017.01.008.
- Scientific Reports 6: 37915, 2016. doi: 10.1038/srep37915.
- Pathology International 66: 678-686, 2016. doi: 10.1111/pin.12472.
- PLOS One 8: e82740, 2013. doi.org/10.1371/journal.pone.0082740
- PLOS One 7: e37220, 2012. doi.org/10.1371/journal.pone.0037220
- Vaccine 28: 780-789, 2010. doi: 10.1016/j.vaccine.2009.10.067.
- J Med Primatol 39: 58-70, 2010. doi: 10.1111/j.1600-0684.2009.00395.x.
- Vaccine 27: 7402-7408, 2009. doi: 10.1016/j.vaccine.2009.08.089.
- Immunology 124: 155-165, 2008. doi: 10.1111/j.1365-2567.2007.02745.x.
- Vaccine 26: 562-572, 2008. doi: 10.1016/j.vaccine.2007.11.031.
- Vaccine 25: 4914-4921, 2007. doi: 10.1016/j.vaccine.2007.04.010.
Therapeutic agents
- Nature Communications, 12:2654, 2021. doi: 10.1038/s41467-021-22964-w
- Antimicrob Agents Chemother 65: e01825-20, 2021. doi: 10.1128/AAC.01825-20.
- Antimicrob Agents Chemother 64: e02561-19, 2020. doi: 10.1128/AAC.02561-19.
- Antiviral Research 178: 104790, 2020. doi.org/10.1016/j.antiviral.2020.104790.
- Antiviral Research 171: 104591, 2019. doi: 10.1016/j.antiviral.2019.104591.
- Antimicrob Agents Chemother 59: 4962-4973, 2015. doi: 10.1128/AAC.00793-15.
- PLOS Pathogens 10: e1004192, 2014. doi: 10.1371/journal.ppat.1004192.
- Antimicrob Agents Chemother 58: 4795-4803, 2014. doi: 10.1128/AAC.02817-14.
- Antimicrob Agents Chemother 55: 4961-4970, 2011. doi: 10.1128/AAC.00412-11.
Pathogenicity of SARS-CoV-2 and development of vaccines and therapeutic agents for COVID-19
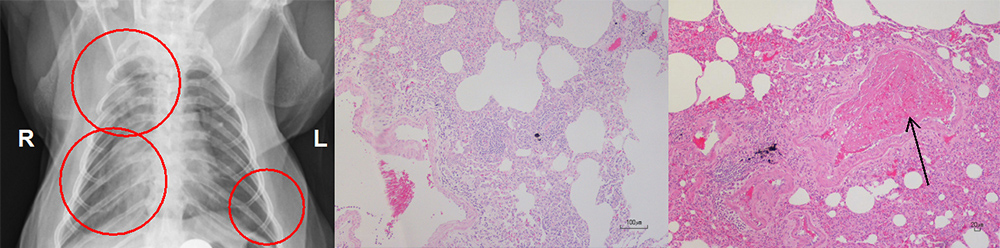
Since 2020, we have been conducting research on the emerging coronavirus, SARS-CoV-2, that causes COVID-19 spreading all over the world. We have shown that SARS-CoV-2 causes pneumonia and thrombus in a nonhuman primate model. The nonhuman primate model of COVID-19 has a lot of similarities to the human disease. Therefore, we use the model for the development of vaccines and therapeutic medicines against COVID-19. Using the nonhuman primate model, we analyze not only viral propagation and genes of SARS-CoV-2 but also pneumonia in X-ray imaging, pathological diagnosis and immune responses against COVID-19.
Articles
Pathogenicity
- J Neuroimmunol 387:578228, 2024. https://doi.org/10.1016/j.jneuroim.2024.578288
- Virology 594:110052, 2024 https://doi.org/10.1016/j.virol.2024.110052
- Virology 554:97-105, 2021. doi.org/10.1016/j.virol.2020.12.013
Vaccines
- Virology 616, 110778, 2026. doi.org/10.1016/j.virol.2025.110778
- Journal of Virology 99: e0229024, 2025. doi:106.10.1128/jvi.02290-24
- Frontiers Microbiol 13:967019, 2022. https://www.frontiersin.org/articles/10.3389/fmicb.2022.967019/full
- bioRxiv. doi: doi.org/10.1101/2021.03.04.433852, posted march 04, 2021.
Therapeutic agents
- iScience 28: 113424, 2025.doi.org/10.1016/j.isci.2025.113424
- Cell Stem Cell 30:1315-1330, 2023.doi.org/10.1016/j.stem.2023.09.001
- iScience 25:105596, 2022. doi.org/10.1016/j.isci.2022.105596
Molecular mechanism of premature aging and senescence
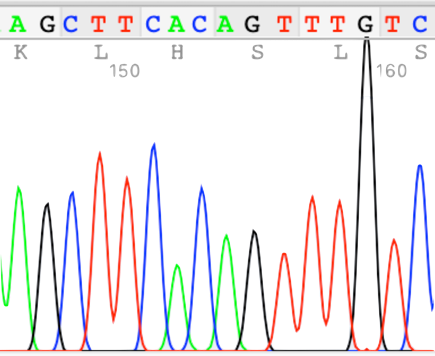
To analyze mechanism of a decline and dysfunction in metabolism and immunity in aging, we have developed a premature aging monkey model using genome-editing technology and an autoimmune monkey model. We examine senescence and development of diseases in the monkey models.
Articles
Autoimmune disease
- HLA: Immune Response Genetics 103: e15316, 2024. doi.org/10.1111/tan.15316
Cancer immunology
We conduct research on cancer immunology using cynomolgus monkeys.
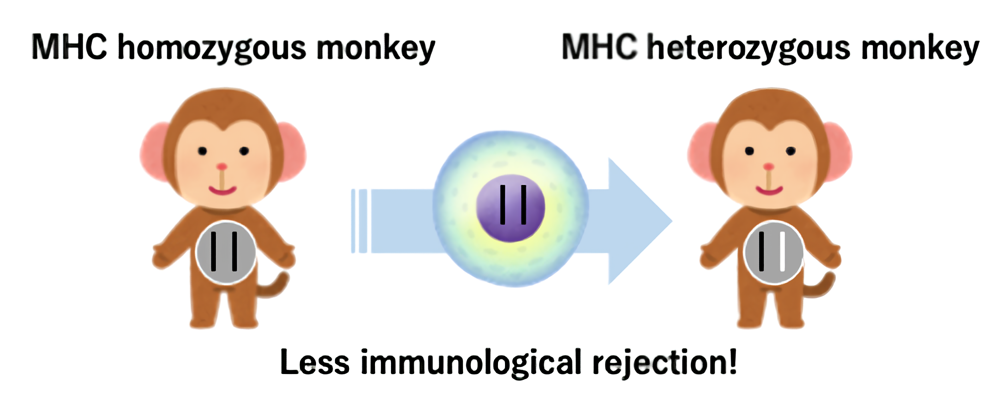
Research Center for Animal Life Science in Shiga University of Medical Science breeds a large number of cynomolgus monkeys. Furthermore, cynomolgus monkeys carrying specific major histocompatibility complex genes (MHC, a kind of blood type) that cause rejection after transplantation are also maintained. Cells from monkeys that have an identical MHC type on both chromosomes (MHC homozygous monkeys) are transplantable to monkeys that have the identical MHC on only one chromosome (MHC heterozygous monkeys) with less immunological rejection (Figure 1). Using the MHC-matched monkeys, we create a monkey cancer model and perform cell transplantation and organ transplantation to develop anti-cancer drugs and drugs to reduce rejection.
We try to create a cancer model of cynomolgus monkeys. Using induced pluripotent stem cells (iPSCs) prepared from MHC homozygous monkey cells, we made cancer cells and transplanted them into MHC heterozygous monkeys. The MHC heterozygous monkeys transplanted with embryonal cancer cells derived from the MHC homozygous monkey iPSCs developed antibodies against GRP94 on the surface of the transplanted cells, and the cancer cells were rejected within 4 weeks (Cancer Res 2017; Figure 2). We also found that cancer cell-specific killer T cells (TILs) infiltrated into the transplanted cancer (Sci Rep 2020).
Currently, we have generated tumor antigen-specific T cells artificially transduced the antigen-specific T cell receptor (TCR) gene of the TILs that attack the embryonal cancer cells, and plan to inject them into the cancer bearing monkey as cell therapy, which are conducted in collaboration with the Department of Molecular Physiological Chemistry (Molecular Therapy - Oncolytics 2022).
Articles
- PLOS One 20, e0305153, 2025.doi.org/10.1371/journal.pone.0305153
- Molecular Therapy - Oncolytics 24:77-86, 2022. doi.org/10.1016/j.omto.2021.12.003
- Scientific Reports 21:8414, 2020. doi: 10.1038/s41598-020-65488-x
- PLOS One 13: e0204745, 2018. doi: org/10.1371/journal.pone.0204745
- Cancer Res 77: 6001-6010, 2017. Doi: 10.1158/0008-5472.CAN-17-0775

 Home
Home